Balancing of Equations| Trial and Error Method | Combustion of Methane | Basic Balancing HD
Balancing of Equations| Trial and Error Method | Combustion of Methane | Basic Balancing Link to my chemistry page http://www.vinstan.com This video is on balancing of equations. The method adopted is called, "Trial and Error method". Law of conservation of matter says, that the mass of reactants, is equal to the mass of products, in a chemical reaction. This is the basis of balancing of equations. Trial and error is the simplest method that can be used for balancing of equations. However, the oxidation number method is a more definitive method for balancing. You will learn about this, in higher classes. The example that we are going to work on today, is the reaction between, methane gas and oxygen gas. This is a combustion reaction, involving three elements, Hydrogen, Oxygen and Carbon. Before we balance the equation, first we have to count the elements, on the reactant side, and the product side. In this case, we have 1 carbon atom, 2 oxygen atoms, and 4 hydrogen atoms, in the reactant side. On the product side, we have 1 carbon atom, two hydrogen atoms, and three oxygen atoms. So the equation is not balanced. Now, we have to pick the elements, that we have to balance first. Is it carbon, oxygen, or hydrogen? The simple rule of thumb would be, pick elements that form single compounds, on the product side first. What I mean by that is, carbon forms only a single product, that is carbon dioxide. Hydrogen forms a single product, that is water or H2O. However, oxygen forms two products. Carbon dioxide contains oxygen, and Water contains oxygen. So we will balance oxygen last. Once you have identified this aspect, you can start the process of balancing. In this case, you can start with either carbon or hydrogen. As both of these elements form only single compounds. I am going to pick Carbon to balance first. On the left side I have, one carbon atom and on the right side I have one carbon atom, so carbon is balanced, at the moment. The next element I am going to balance is hydrogen. There are four hydrogen on the left, and only two hydrogen on the right. In order to balance the hydrogen atoms, I will add a coefficient, to the water molecule on the product side, this will make the number of hydrogens 4. The number that you add before a molecule, is called a coefficient. Now that we have balanced both Carbon and Hydrogen on the reactant side and product side, we will now attempt to balance the oxygen atoms. If you count the oxygen atoms, in the product side, you will see that, there are two oxygen atoms in carbon dioxide, and two atoms of oxygen in two water molecules. This makes the total number of oxygen atoms four, in the product side. On the reactant side, we have oxygen as elemental diatomic oxygen. The number two after oxygen, is called a subscript. In order to make the oxygen atoms four on both side, we will add a coefficient of 2 to the diatomic oxygen molecule, on the reactant side. Now I have 4 oxygen atoms in the reactant side.
 HD
HD HD
HD![How to squeeze extra 57HP out of 3.8L Pajero! [Dyno Testing]](https://i.ytimg.com/vi/bjNuR69v6xw/mqdefault.jpg) HD
HD HD
HD
 HD
HD

 HD
HD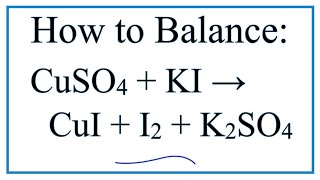 HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD

 HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD HD
HD