CHEMISTRY 201: Calculating Boiling Point Using Clausius Clapeyron Equation HD
In this example problem, we calculate the boiling point of methanol using the Clausius Clapeyron equation. The normal boiling point of methanol is 64.7 degrees Celsius. Calculate the boiling point of methanol at an elevation of 4000 m above sea level where the pressure is 478 mmHg. The heat of vaporization for methanol is 35.3 kJ/mol.
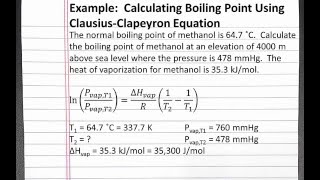 HD
HD HD
HD HD
HD HD
HD HD
HD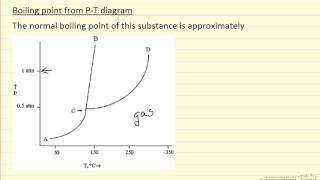 HD
HD HD
HD HD
HD HD
HD HD
HD